29-Nov-1978
Biocon’s Founding Day

Incorporation certificate 29th November 1978
Biocon India Pvt. Ltd. is incorporated as a joint venture between Kiran Mazumdar-Shaw and Biocon Biochemicals Ltd. Ireland. Starting with three employees in a rented shed in Koramangala, suburban Bangalore. Biocon began manufacturing and exporting Papain, a plant enzyme, and Isinglass, a marine hydrocolloid – both, key products for the brewing industry.
1979
First export of Papain, a plant enzyme derived from Papaya

Kiran Mazumdar-Shaw conducts a business meeting with a Papain supplier
Biocon’s first export was Papain, a plant enzyme derived from Papaya, to U.S. and Europe, signalling the company's ambition to produce high quality, world class products in India for global markets.
1980
Biocon’s ground breaking of its heritage campus on 20th KM, Hosur Road, Bangalore

Kiran Mazumdar-Shaw and Les Auchincloss at the foundation laying ceremony for Biocon’s first building on Hosur Road, Bangalore
Biocon acquires a 20-acre plot on Hosur Road, Bangalore for INR 600,000 to expand operations. On March 8th, 1980, the foundation stone was laid for the building that currently houses the company’s corporate office.
1981
Biocon starts construction at its own site

Kiran Mazumdar-Shaw at the Biocon building site.
Biocon decides to move beyond its frugal operations in rented premises and starts building its own integrated campus at 20th KM, Hosur Road, Electronics City, Bangalore.
02-Nov-1983
Biocon’s Bangalore campus is inaugurated

Kiran Mazumdar-Shaw with dignitaries, including Irish ambassador H.E. Bernard McHugh, at the inauguration of Biocon’s new campus.
Biocon’s new campus at 20th KM, Hosur Road, Electronics City, Bangalore is inaugurated by Irish Ambassador H.E. Bernard McHugh. Manufacturing activity shifted from the rented shed to the new site. R&D was also initiated.
1984
R&D division established to develop Solid State Enzyme Fermentation Technology

Enzyme manufacturing using proprietary Solid State Fermentation Technology.
Biocon established its R&D division, which initially focused on the development of solid state fermentation technology to develop novel enzymes.
1989
Unilever Acquires Biocon’s Irish Parent

Unilever Acquires Biocon’s Irish Parent
Biocon Biochemicals Ltd, Ireland, including its subsidiaries, are acquired by Unilever Plc. and merged with its wholly-owned subsidiary Quest International, The Netherlands.
Biocon India begins producing enzymes for Unilever’s food businesses.
1990
Scaling up indigenous fermentation technology

RK Hedge, Former Chief Minister, Karnataka, inaugurates Biocon’s enzyme fermenter at Biochemizyme
Biocon scales up its in-house research program based on a proprietary solid state fermentation technology through a new subsidiary Biochemizyme Ltd to scale up from pilot to plant level.
1993
First Life Sciences Company from India to get ISO 9001 Certification

First Life Sciences Company from India to get ISO 9001 Certification
Biocon's R&D and manufacturing facilities receive ISO 9001 certification from RWTUV, Germany, making it the first life sciences company in India to get the ISO 9001 Certification.
1994
Biocon establishes Syngene International Pvt. Ltd

Dr Gordon Ringold with Kiran Mazumdar-Shaw and Goutam Das, COO, Syngene at the inauguration of Syngene
Syngene is incorporated as a Custom Research Organisation (CRO) in 1994 to provide efficient, high-quality, cost-effective research & development services in chemistry and biology to the global pharmaceutical industry.
1995
Biocon Quest India Ltd (BQIL) is established

Dr. Sergio Lecchini, CEO of Quest International, with Kiran Mazumdar-Shaw and other dignitaries at the inauguration of BQIL
Unilever acquires 50% stake in Biochemizyme and creates Biocon Quest India Ltd (BQIL), with Biocon and Unilever holding 50% stake each in the entity. A four-fold expansion of Biochemizyme’s solid state fermentation capacity aims to produce a range of fungal enzymes for the food and pharmaceutical industry.
1998
Biocon becomes an independent entity

Kiran Mazumdar-Shaw and John Shaw take full ownership of Biocon
Kiran Mazumdar-Shaw and John Shaw buy out Unilever’s share in Biocon India Ltd. making it an independent, privately owned company
09-Jan-1999
Nobel Laureate Prof. James D Watson visits Biocon

Nobel Laureate Prof. James D Watson visits Biocon.
Nobel Laureate Prof. James D. Watson, remembered for co-discovering DNA’s double-helix structure, visits Biocon.
2000
Clinigene - India's first Clinical Research Services Organisation

Henri Temeer, President, Genzyme with Kiran Mazumdar-Shaw at the inauguration of Clinigene
Biocon establishes Clinigene as India's first Clinical Research Organisation to develop world-class capabilities for clinical research and related activities. Clinigene, a 100% subsidiary of Biocon, starts providing clinical research services to domestic and multinational companies.
2000
Biocon’s proprietary bioreactor, PlaFractor, is commissioned

Prof. Charles Cooney inaugurates the PlaFractor plant
PlaFractor, Biocon’s patented fermentation reactor, is commissioned. The indigenous PlaFractor fermentation platform proved a commercial success as Biocon was the first company to make Mycophenolate Mofetil using this technology in 2000.
2000
First fully automated submerged fermentation plant commissioned

Inside view of Submerged Fermentation Plant
Biocon commissions its first fully automated submerged fermentation plant to produce specialty pharmaceuticals
2001
First company globally to be approved by U.S. FDA to sell Lovastatin API

Solid state fermentation production of Lovastatin
Biocon is the first company globally to get U.S. FDA approval for making Lovastatin API through an innovative solid state fermentation technology.
2001
Patent granted for proprietary PlaFractor technology

The indigenous PlaFractor technology proved a commercial success
Biocon’s proprietary bioreactor PlaFractor is granted a U.S. and worldwide patent.
2002
Biocon Biopharmaceuticals established as a JV with CIMAB, Cuba

Biocon Biopharmaceuticals established as a JV with CIMAB, Cuba
Biocon establishes a joint venture company, Biocon Biopharmaceuticals Pvt Ltd, with CIMAB S.A., a Cuban biotechnology company, to manufacture biologicals developed by CIMAB, including Nimotizumab & Itolizumab monoclonal antibodies.
2004
World's first Pichia-based rh-Insulin developed and commercialised in India
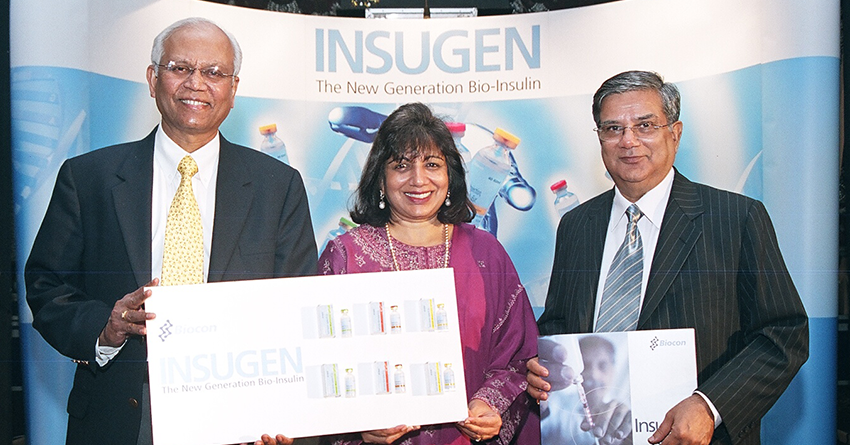
The launch of Insugen®, an indigenous brand of rh-insulin, helped lower insulin prices in India
Biocon is the first company in the world to commercialise recombinant human insulin (rh-insulin), Insugen®, using a novel Pichia pastoris yeast expression system. Today, Biocon’s rh-insulin is approved in over 40 countries.
2004
Biocon makes a resounding debut on the Indian stock market

Kiran Mazumdar-Shaw at the National Stock Exchange, Mumbai on the day Biocon went public
Biocon creates a buzz in the stock market in March 2004 with its hugely successful IPO. The IPO received bids thirty-three times the 1 crore shares available for subscription during the bidding process. On Day 1 of listing on the bourses, Biocon closes with a market value of USD 1.1 billion, making Biocon only the second Indian company to cross the USD1 billion mark on the first day of listing.
06-Jun-2006
Biocon Park, India’s first private Biotechnology SEZ, inaugurated

Dr APJ Abdul Kalam, Former President of India, inaugurates Biocon Park on June 6, 2006
Former President of India, Dr APJ Abdul Kalam, inaugurates Biocon Park, India's largest integrated biotechnology hub, comprising an integrated cluster of research laboratories and manufacturing facilities spread across 90 acres.
External Link to Address by Dr APJ Abdul Kalam during Inauguration of Biocon Park
2006
First company in India to launch a novel biologic, Nimotuzumab

Bollywood actor Shahrukh Khan at the launch of BIOMAb EGFR®
Biocon earns the distinction of being the first company in India to launch a novel biologic, Nimotuzumab, for head and neck cancer patients. Through the introduction of this molecule as BIOMAb EGFR®, Biocon enhanced the treatment outcome as well as quality of life of cancer patients in India.
2006
India's largest multi-product Biologics facility inaugurated

India's largest, state-of-the-art multi-product Biologics facility inaugurated
Biocon Biopharmaceuticals, India's largest, state-of-the-art multi-product Biologics facility, inaugurated at Biocon Park for manufacturing a range of products encompassing monoclonal antibodies and other recombinant therapeutics. P. Chidambaram, former Union Minister of Finance, inaugurated the facility on April 17, 2006.
2007
Biocon divests enzymes business

Biocon divests its legacy enzymes business to Novozymes for USD 115 million to increase focus on developing, manufacturing biopharmaceuticals.
2008
Biocon acquires stake in AxiCorp GmbH, a German pharmaceutical company

Strategic investment in AxiCorp enables Biocon to establish its first presence in Europe to market its biopharmaceuticals
Biocon acquires a 70% stake in German pharmaceutical company, AxiCorp GmbH, for a consideration of EUR30 million to market and distribute a range of biopharmaceuticals in Germany and the rest of Europe. Biocon divested its stake in AxiCorp in 2011.
2009
Biocon, Mylan enter strategic global partnership for development of biosimilars

Biocon and Mylan signed one of the earliest partnerships in the global biosimilars space
Biocon partners with U.S.-based Mylan for the co-development of a high-value portfolio of biosimilars for oncology and autoimmune indications. The portfolio initially included three monoclonal antibodies — Trastuzumab, Bevacizumab and Adalimumab — and two recombinant proteins — Pegfilgrastim and Etanercept. It marked one of the earliest partnerships in the global biosimilars space.
2009
Biocon launches Basalog® (Insulin Glargine) in India

Kiran Mazumdar-Shaw at the launch of Basalog® (Insulin Glargine) in India
Biocon launched Insulin Glargine under the brand name Basalog® in India, providing diabetes patients with an advanced, affordable insulin therapy.
2009
Biocon Bristol-Myers Squibb Research Centre (BBRC) inaugurated

Dr M.K. Bhan, Former Secretary, Department of Biotechnology, India, inaugurates the BBRC building
Syngene inaugurates a fully dedicated research and development facility for Bristol-Myers Squibb at Biocon Park.
2010
Biocon and Pfizer enter into a strategic global agreement

Kiran Mazumdar-Shaw and Pfizer's David Simmons at the signing of a global collaboration agreement
Biocon and Pfizer enter into a strategic global agreement for the worldwide commercialisation of Biocon's biosimilar versions of Insulin and Insulin analog products: Recombinant Human Insulin, Glargine, Aspart and Lispro. Biocon and Pfizer amicably dissolved their global partnership in 2012 and all rights licensed to Pfizer for these insulin molecules reverted to Biocon.
2011
Biocon starts work on the largest integrated insulins facility in Asia

Kiran Mazumdar-Shaw at the unveiling of the plaque commemorating the initiation of the Malaysia insulin facility project
Biocon announces the project commencement of its state-of-the-art insulin manufacturing and R&D facility, the largest integrated insulin facility in Asia, at Bio-XCell in Johor, Malaysia.
2011
Biocon launches INSUPen® for patients in India

Biocon launches INSUPen®, a convenient, affordable and reusable insulin pen, for patients in India
Biocon launched in India its world-class, reusable insulin delivery device INSUPen® designed for efficiently, accurately and safely delivering both Basalog and Insugen, thus maximising patient convenience.
2012
Biocon Research Centre Inaugurated

Noble Laureate Prof Kurt Wuthrich inaugurates the Biocon Research Centre
Biocon inaugurated a state-of-the-art Biocon Research Centre, spread across 200,000 sq ft, as part of its aspiration to build a centre of research excellence to accelerate a culture of innovation to promote path breaking research for biopharmaceuticals.
2013
World’s first anti-CD6 monoclonal antibody Itolizumab commercialised in India

The Biocon team that was involved in taking Itolizumab from ‘lab to market’
Biocon launched Itolizumab, a novel, first-in-class humanised anti-CD6 monoclonal antibody approved for treating psoriasis, under the brand name ALZUMAb™ in India. This is the first anti-CD6 monoclonal antibody in the world to be commercialised, which makes it a path breaking innovation to come out of an Indian laboratory.
07- March-2013
Biocon House inaugurated

Yamini Mazumdar, Kiran Mazumdar-Shaw and John Shaw at the inauguration of Biocon House
Biocon House, which now houses Biocon Biologics India Ltd, is inaugurated at Semicon Park, Electronic City, Bangalore
2013
Biocon establishes Biocon Academy under its CSR agenda

Biocon Academy is established as a Centre of Excellence for Advanced Learning in Applied Biosciences
Biocon establishes Biocon Academy as a Centre of Excellence for Advanced Learning in Applied Biosciences under its Corporate Social Responsibility (CSR) agenda as part of the company’s commitment to create a globally competitive biotech ecosystem in India through skill development.
2014
Biocon introduces world’s first biosimilar Trastuzumab, CANMAb™, in India

Biocon introduces world’s first biosimilar Trastuzumab, CANMAb™, in India
Biocon introduces CANMAb™ in India as the world’s lowest priced Trastuzumab, opening the doors for HER2-positive metastatic breast cancer patients to access an affordable therapy.
2015
Syngene debuts on the Indian stock market

Kiran Mazumdar-Shaw, Peter Bains and other dignitaries at the debut of Syngene on the Indian stock market.
Biocon’s research services subsidiary, Syngene, debuts on the Indian stock market. Syngene’s market capitalisation crossed USD 1 billion within a week of listing.
2015
Biocon’s new insulin devices facility inaugurated in Bangalore

Kiran Mazumdar-Shaw with Amitabh Kant, CEO of NITI Ayog, at the inauguration of the state-of-the-art new insulin devices facility in Bengaluru
Biocon inaugurates a state-of-the-art 100,000 square feet facility in Bangalore for manufacturing new generation, patient-friendly devices for its insulins portfolio.
Biocon also launches Basalog One, a high-end, pre-filled Insulin Glargine pen that is almost painless, safe and convenient to administer.
2016
Biocon Gets its First Generic Formulation Approval in EU

Rosuvastatin is the first Generic Formulation product to be approved in EU
Biocon receives European approval for its generic Rosuvastatin Calcium tablets. This is Biocon's first Generic Formulations approval in the regulated markets and marks an important milestone in the company’s forward integration from generic APIs to generic finished dosages.
2016
First biosimilar from India to be approved in Japan

Kiran Mazumdar-Shaw announces the approval of biosimilar Insulin Glargine in Japan
Biocon achieves a major regulatory milestone when Japan's health regulator approves the sale of its biosimilar Insulin Glargine, partnered locally with FUJIFILM Toyama Chemical. This is Biocon’s first biosimilar approval in a developed market and the first biosimilar from a company in India to be approved in Japan.
2017
First company from India to get U.S. FDA approval for a biosimilar

Celebrations at Biocon to mark the U.S. FDA approval of biosimilar Trastuzumab
Biocon and Mylan create history by becoming the first companies globally to receive U.S. Food and Drug Administration (FDA) approval for biosimilar Trastuzumab. Ogivri™ (trastuzumab-dkst) is the first biosimilar from Biocon and Mylan’s joint portfolio approved in the U.S. and Biocon is the first Indian company to receive a U.S. FDA approval for a biosimilar.
2017
Biocon’s first overseas facility in Malaysia starts operations

Biocon commences operations at its first overseas insulins manufacturing facility in Malaysia
Biocon starts commercial operations at its first overseas insulins manufacturing facility in Malaysia. Biocon’s rh-Insulin is the first locally manufactured biosimilar product to be approved for sale by the National Pharmaceutical Regulatory Authority (NPRA), Malaysia.
2017
Biocon outlicenses Itolizumab to Equillium for U.S., Canada

Biocon outlicenses Itolizumab to Equillium for U.S., Canada
Biocon licensed Itolizumab for the U.S. and Canada markets to San Diego-based biotechnology company Equillium through an equity stake. Later, it expanded the scope of the licensing agreement with Equillium for Itolizumab, to include Australia and New Zealand.
2017
Biocon launches its biosimilar Bevacizumab in India
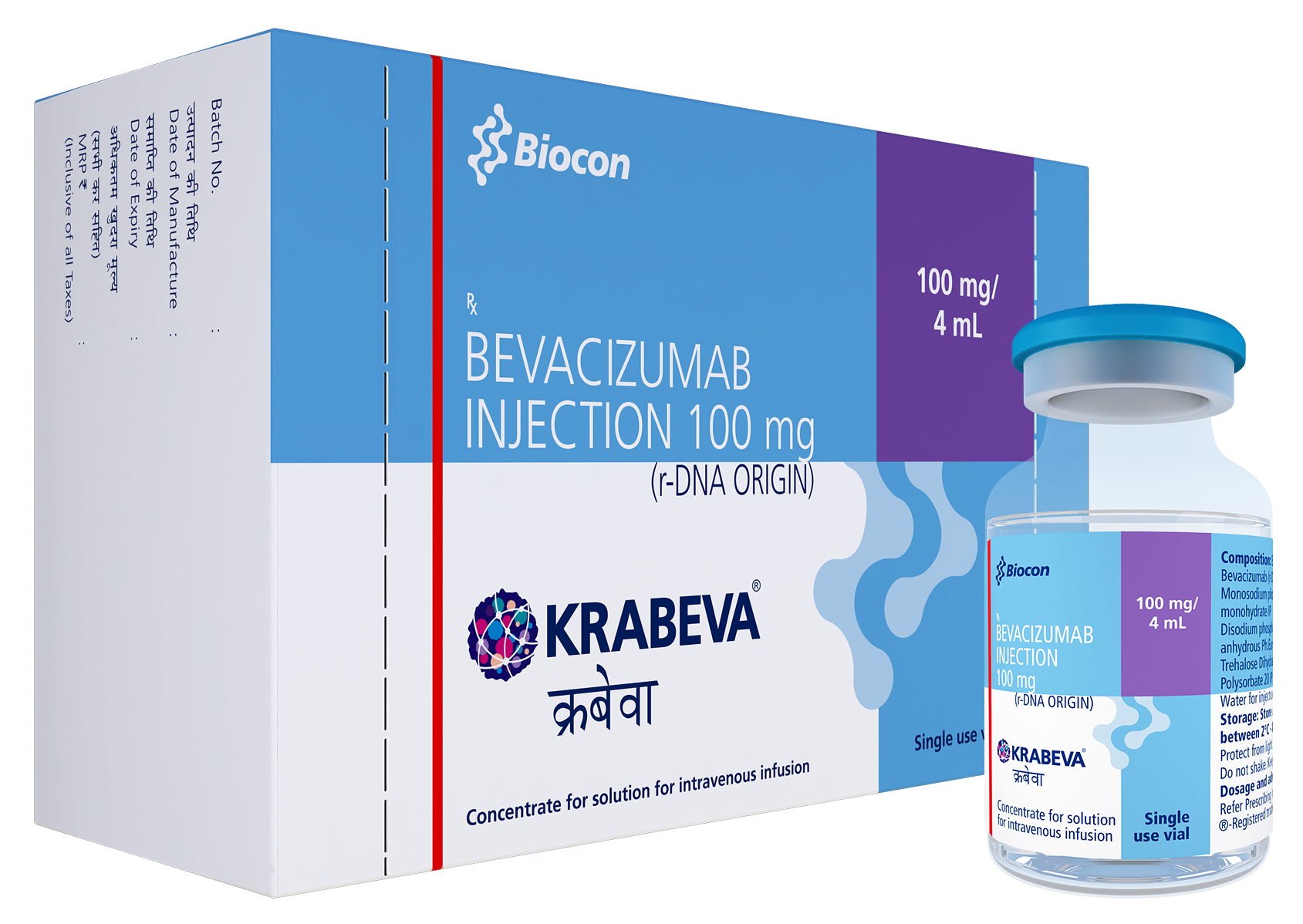
Biocon launches its biosimilar Bevacizumab in India
Biocon successfully launches its biosimilar Bevacizumab in India as KRABEVA® for patients of various types of cancer. KRABEVA®, the second oncology biosimilar launched in India after CANMAb (Trastuzumab), is prescribed for metastatic colorectal cancer (mCRC) and several other types of lung, kidney, cervical, ovarian and brain cancers.
2018
First company from India to have biosimilar commercialised in U.S.

Biocon achieves another milestone with the approval and launch in the U.S. of Fulphila™, biosimilar Pegfilgrastim co-developed with partner Mylan. Biocon is the first company from India to have a biosimilar commercialised in the U.S.
2018
Semglee is the first biosimilar from Biocon & Mylan’s joint insulins portfolio to be approved in Europe

Biocon and Mylan’s biosimilar Insulin Glargine, Semglee, receives European approval for commercialisation.
2018
Biocon, Sandoz enter global partnership for next-gen biosimilars

Biocon enters into a global partnership with Sandoz, a Novartis division and a global player in biosimilars to develop a next-generation biosimilars portfolio, which will help patients worldwide gain access to a range of high quality, affordable immunology and oncology biologics.
2018
Two biosimilars get marketing authorisation in EU

Fulphila®, a biosimilar Pegfilgrastim jointly developed by Biocon and Mylan, is granted Marketing authorisation by the European Commission. Ogivri®, a biosimilar Trastuzumab jointly developed by Biocon and Mylan, also gets EC’s nod.
2019
Biosimilars business consolidated under independent entity Biocon Biologics

Biocon consolidates the development, manufacturing and commercialisation operations of its biosimilars business under an independent entity, Biocon Biologics, with its own dedicated management.
2019
Ogivri, biosimilar Trastuzumab, commercialised in U.S
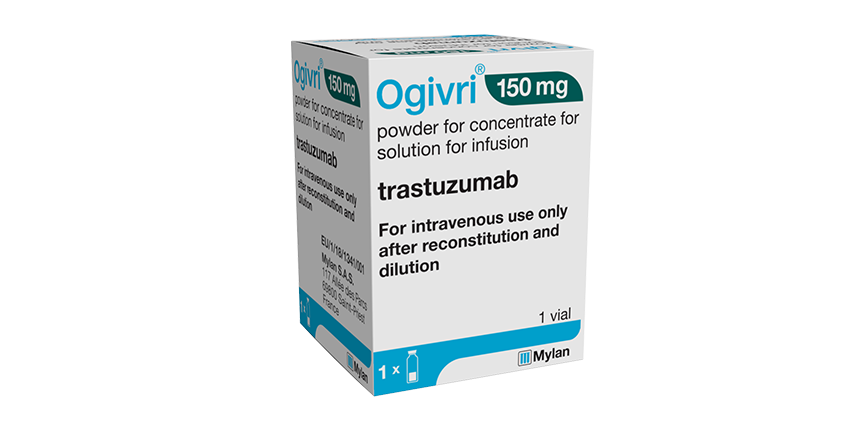
Biocon becomes the first Indian player to launch biosimilar Trastuzumab, Ogivri, in the U.S. through partner Mylan.
2019
Bicara Therapeutics incorporated to advance novel immuno-oncology assets

Bicara Therapeutics is incorporated as a wholly owned subsidiary of Biocon in Boston, U.S., to focus on developing a pipeline of bi-functional antibodies that exploit the advances in immuno-oncology.
2020
True North makes a primary equity investment in Biocon Biologics

Biocon Biologics approves a primary equity investment by True North, starting the value unlocking process for Biocon's biosimilars subsidiary.
2020
Itolizumab Gets DCGI Nod for Restricted Emergency Use in Moderate to Severe COVID-19 Patients
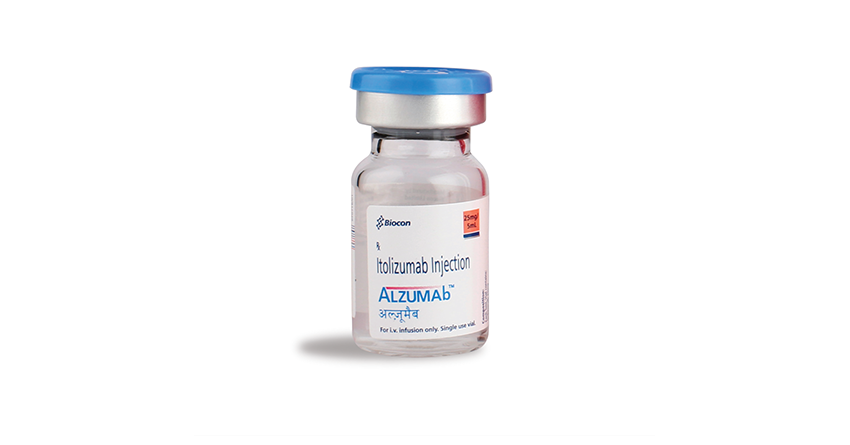
The Drugs Controller General of India (DCGI) approves 'restricted emergency use' of Itolizumab (ALZUMAb®) in India, a repurposed drug, for the treatment of cytokine release syndrome (CRS) in moderate to severe ARDS (acute respiratory distress syndrome) patients due to COVID-19.
2020
Tata Capital picks up minority stake in Biocon Biologics

Tata Capital Growth Fund makes a primary equity investment for a minority stake in Biocon Biologics.
2020
Semglee™ (insulin glargine injection) Launched in U.S.

Biocon Biologics and partner Viatris achieve the key milestone of obtaining U.S. FDA approval and bringing to market both the vial and pen presentations of Semglee (insulin glargine injection), the first for any company following the reference product.
2020
Biocon Biologics Receives USD 150 Million Capital Injection from Goldman Sachs

Transaction Values Biocon Biologics at USD 3.94 Billion
2021
Biocon Biologics Receives USD 75 Million Capital Injection from ADQ

Transaction Values Biocon Biologics at ~USD 4.17 Billion
2021
Biocon Biologics Partners with Adagio Therapeutics to Advance Antibody for the Prevention and Treatment of COVID-19

U.S. based Adagio Therapeutics has granted an exclusive license to Biocon Biologics to manufacture and commercialize an antibody treatment based on ADG20 for India and select emerging markets.
2021
Semglee® Receives Historic U.S. Approval for First Interchangeable Biosimilar

Semglee® Pen
Biocon Biologics and partner Viatris receive the world’s first interchangeable biosimilar approval from U.S. FDA for Semglee (Insulin Glargine) for treating diabetes. The historic approval is a milestone achievement for both companies.
2021
Biocon Biologics and Serum Institute Life Sciences Enter Strategic Alliance

Kiran Mazumdar-Shaw with Mr. Adar C. Poonawalla
Biocon Biologics enters a strategic alliance with Serum Institute Life Sciences (SILS) with the objective of addressing inequitable access for life-saving vaccines and biologics in both emerging and developed markets. Biocon Biologics to get committed access to 100 million doses of SILS’ vaccines annually for 15 years in exchange for ~15% stake at a post-money valuation of ~USD 4.9 billion.
2021
Biocon Enters Prestigious Dow Jones Sustainability Emerging Markets Index

Biocon Ltd has made it to the DJSI EM Index with a Total Sustainability Score of 45 as against an industry average of 18, achieving a 93rd percentile position. We are among the Top 15 companies from India and one of the 12 companies from the Pharmaceuticals, Biotechnology & Life Sciences sectors to be featured in the index for 2021.
2022
Biocon Biologics Inks Deal to Acquire Viatris’ Global Biosimilars Business

Biocon Biologics Ltd agrees to acquire partner Viatris’ global biosimilars business to create a unique global, vertically integrated biosimilars leader. This transformational deal prepares Biocon Biologics for the long-term value creation for all its stakeholders.
2022
Biocon Biologics Completes Acquisition of Viatris’ Global Biosimilars Business

Biocon Biologics Ltd. has successfully completed its multi-billion dollar acquisition of the global biosimilars business of its partner Viatris Inc. This acquisition builds on Biocon’s long-standing, strategic partnership with Viatris and is a historic milestone in Biocon Biologics’ value creation journey.
2023
Biocon Biologics launches HULIO®

Biocon Biologics launches HULIO®, a biosimilar to Humira® (adalimumab), in the U.S. for patients with certain inflammatory diseases. This is Biocon Biologics’ 4th biosimilar in the U.S.
2023
Biocon Biologics Concludes Integration in ~120 Countries
Biocon Biologics completes transition of the acquired biosimilars business from Viatris in ~120 countries. This transforms Biocon Biologics into a unique, fully integrated ‘lab to market’ biosimilars company.
2024
Biocon Biologics Achieves $1 Billion Annual Revenue Milestone

Biocon Biologics crossed the annual revenue milestone of USD 1 billion as it strategically consolidated business operations, improved market share for products, especially in the U.S., and expanded geographical reach.
2024
Biocon Biologics Secures $1.1 Billion Refinancing with Asia-Pacific’s First Biopharma USD Bonds

Biocon Biologics successfully refinances $1.1 billion in long-term debt through a combination of USD bonds ($800 million) and a new syndicated debt facility. This debut USD bond issuance by Biocon Group is notable as the first by a biopharmaceutical company in the Asia-Pacific region and the largest debut issuance from a high-yield rated issuer in India over the past decade.
2024
Biocon Limited Achieves Milestone with UK Approval for gLiraglutide

Biocon Limited secured regulatory approval for gLiraglutide in the UK; the first in a major regulated market, and among the several ‘firsts’ achieved by Biocon Group in the biopharmaceuticals domain.
2025
Biocon Limited Launches gLiraglutide for Diabetes and Obesity in the UK

The launch of gLiraglutide in the UK marks a significant milestone for Biocon Limited in its mission to expand access to affordable and high-quality diabetes and obesity treatments.
2025
Biocon Biologics Launches YESINTEK™ for Patients in U.S.

Biocon Biologics' launch of YESINTEK™ (ustekinumab-kfce) in the U.S. is a significant step in enhancing patient care for inflammatory conditions and increasing access to biosimilars. It is among the first Stelara® (ustekinumab) biosimilars available in the U.S. market.

