The world’s first novel anti-CD6 monoclonal antibody
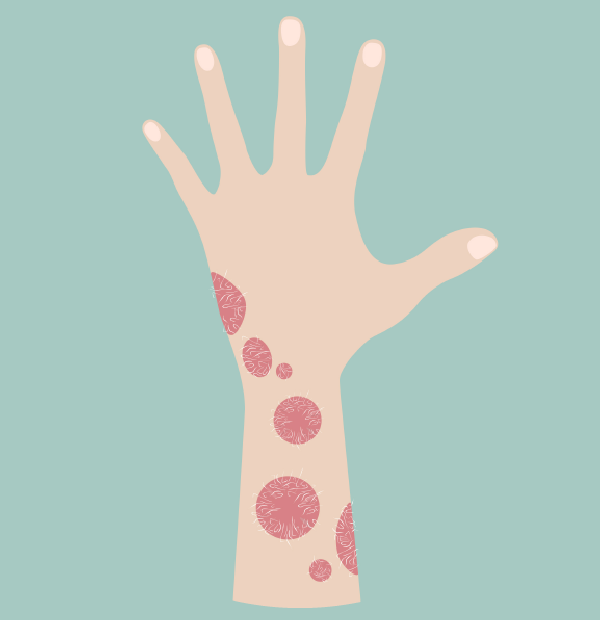
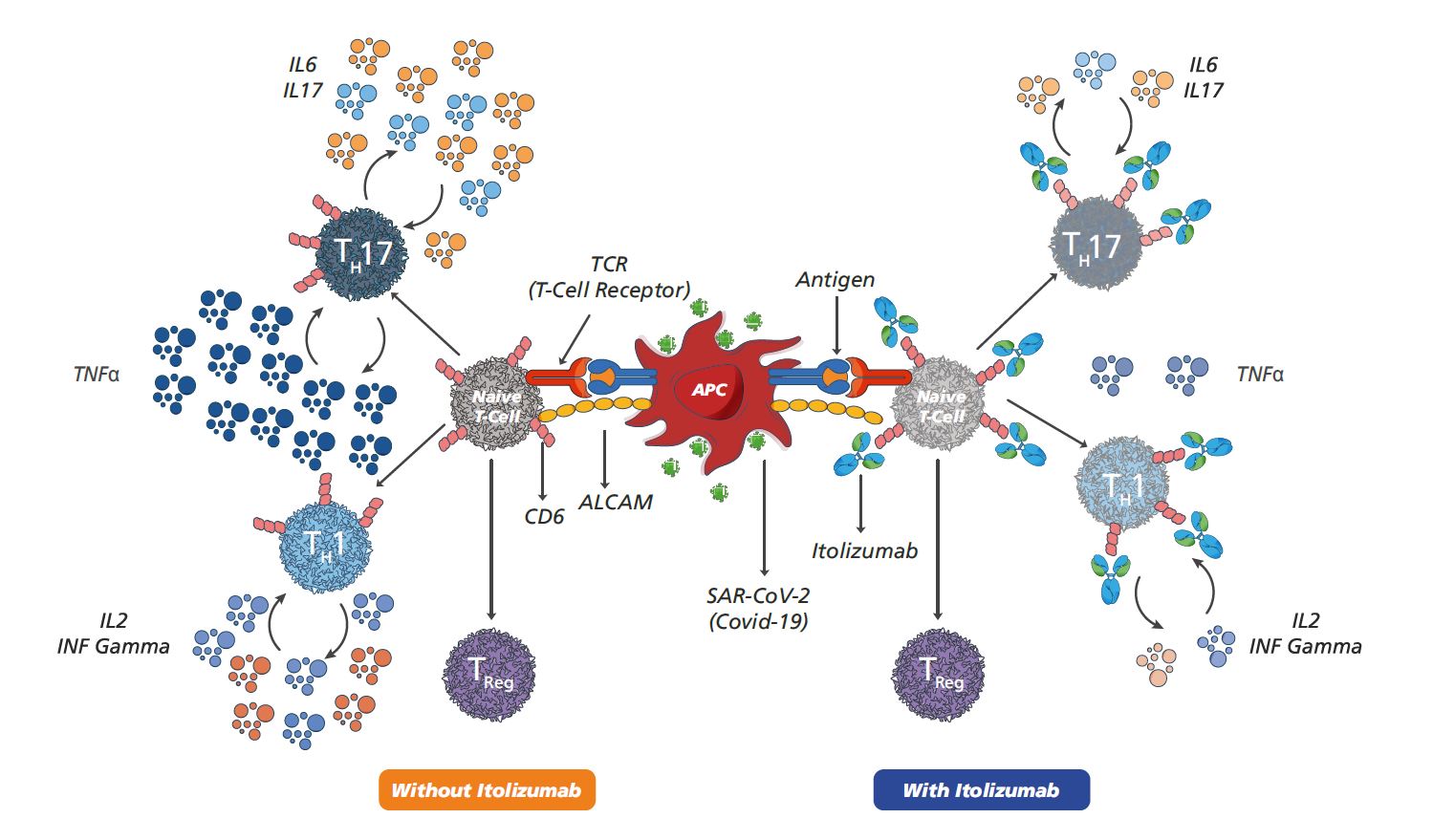
Itolizumab was launched in India in 2013 under the brand name ALZUMAb™. Itolizumab is Biocon’s second ‘lab to market’ novel biologic after Nimotuzumab and offers a ‘best-in-class’ biologic drug for acute psoriasis. ALZUMAb™ offers a less aggressive dosing regimen and a longer treatment free period and has seen encouraging outcomes. Our research also indicated Itolizumab’s potential in treating several autoimmune conditions.
Itolizumab has received emergency use approval in India to treat cytokine release syndrome in COVID-19 patients with moderate to severe acute respiratory distress syndrome.