Biosimilars Up 34%; Research Services Up 26%; Generics Up 18%
Bengaluru, Karnataka, India: November 14, 2022:
Biocon Ltd (BSE code: 532523, NSE: BIOCON), an innovation-led global biopharmaceuticals company, today announced its consolidated financial results for the second quarter ended September 30, 2022.
Leadership Comments
BIOCON GROUP
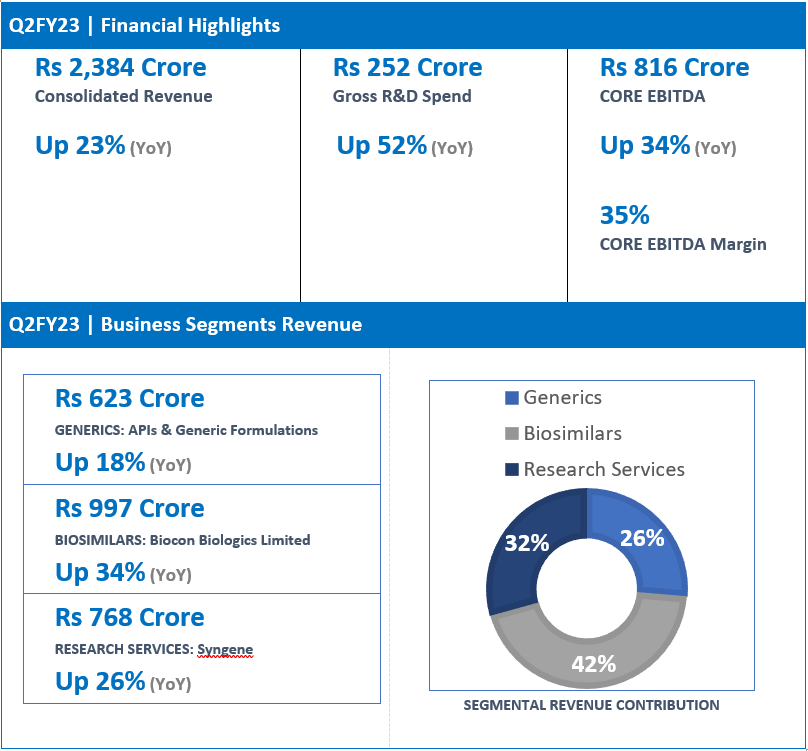
“We reported a strong consolidated revenue growth of 23% YoY for Q2FY23 at Rs 2,384 Crore driven by 34% growth in Biosimilars, 26% in Research Services and 18% in the Generics business. Our Gross R&D spends increased by 52% YoY this quarter to Rs 252 Crore reflecting our advancing pipeline that will drive our future growth. Core EBITDA was up by 34% at Rs 816 Crore, representing healthy core operating margins of 35% versus 33% in the same quarter last year.
“We have delivered a resilient performance in H1FY23, with all segments delivering strong revenue growth. We expect to consolidate on this performance in the second half of FY23. Enhanced capacities and new launches will drive growth for our API and Generic Formulations business, while continued business momentum should help Syngene achieve its guidance for the full year.
“The consolidation of Viatris’ global biosimilars business and the strategic vaccines alliance with Serum Institute will add to the growth of the Biosimilars business in H2FY23. We have secured necessary financing and obtained relevant approvals for the Viatris transaction, which is expected to close shortly.”
— Kiran Mazumdar-Shaw, Executive Chairperson, Biocon and Biocon Biologics
BIOCON GENERICS
“I am pleased with the sequential as well as YoY growth of the Generics business in Q2, on the back of the stabilization of our API business, with immunosuppressants being a significant contributor. Our statins portfolio continued to encounter pricing pressures.
“Our Generic Formulations business delivered a healthy performance in the quarter, with both base business as well as new product launches gaining traction. The business also secured several key approvals for our vertically integrated products in the EU and the rest of the world (RoW) markets, providing further impetus to our geographical expansion plans in the quarters ahead.
— Siddharth Mittal, CEO & Managing Director, Biocon
BIOCON BIOLOGICS
“Biocon Biologics maintained a healthy operating performance in Q2FY23 with revenues increasing 34% YoY to Rs 997 Crore, driven by higher sales of our biosimilar insulins and antibodies in advanced and emerging markets. We have seen an uptick in new prescription share for Semglee and volume market share for Fulphila in the U.S. Core EBITDA at Rs 449 Crore was up 48% YoY, representing strong margins of 46%. R&D investments at Rs 184 Crore, an increase of 142% YoY, reflects the good progress of our pipeline laying the foundation for future growth of our business.
“In preparation for a direct commercial presence in the advanced markets, we have made key leadership appointments this quarter. We believe the conclusion of the strategic transactions with Viatris and Serum Institute, expected to be closed in Q3FY22, will position Biocon Biologics as a fully integrated, leading global biosimilars player.”
— Dr Arun Chandavarkar, Managing Director, Biocon Biologics
SYNGENE
“Our performance in the first half of the year was good. We started the year with strong momentum and delivered a robust first quarter. Performance in the second quarter was ahead of market expectations. All our divisions performed well and revenue from operations grew by 26%. We continue to see good demand for our services, which has helped us deliver strong revenue growth and puts us on a solid track for the rest of the year.”
— Jonathan Hunt, CEO & Managing Director, Syngene
Awards & Recognitions
- Biocon (including Biocon Biologics) improved its ESG score to 52 from 45 in the previous year in the 2022 S&P Global Corporate Sustainability Assessment released in October on the back of key initiatives undertaken during the past year.
- Biocon (including Biocon Biologics) has been named among the Top 10 global employers in the biotech, pharma and biopharma sectors by the U.S.-based prestigious Science magazine Biocon has been in the Top 20 global biotech employers ranking since 2012.
- Biocon Biologics has been recognized for world class Intellectual Property (IP) management and IP value creation in the Asia-Pacific region and included on the prestigious Asia IP ELITE list for 2022.
FINANCIAL HIGHLIGHTS (CONSOLIDATED): Q2FY23
| Particulars | Q2FY23 | Q2FY22 | YoY (%) |
| INCOME | |||
| Generics | 623 | 530 | 18% |
| Biosimilars | 997 | 743 | 34% |
| Novel Biologics | – | 12 | (100)% |
| Research services | 768 | 610 | 26% |
| Inter-segment | (69) | (54) | 26% |
| Revenue from operations # | 2,320 | 1,840 | 26% |
| Other income | 65 | 105 | (39)% |
| Total Revenue | 2,384 | 1,945 | 23% |
| R&D Expenses in P&L | 242 | 146 | 65% |
| Gross R&D Spend | 252 | 165 | 52% |
| EBITDA | 535 | 551 | (3)% |
| EBITDA Margins | 22% | 28% | |
| Core EBITDA* | 816 | 609 | 34% |
| Core EBITDA Margins* | 35% | 33% | |
| PBT (before Exceptional Items) | 246 | 276 | (11)% |
| PBT | 229 | 206 | 11% |
| Net Profit (before Exceptional Items^) | 168 | 188 | (10)% |
| Net Profit Margins (before Exceptional Items^) | 7% | 10% |
Figures above are rounded off to the nearest Crore; % based on absolute numbers.
#Includes Licensing income. *Core EBITDA is EBITDA net of R&D expense, licensing, forex, dilution gain in Bicara, mark-to-market movement on investments.
^ Exceptional items during Q2 FY23 comprise MAT credit balance charge of ₹107 Cr on adoption of new tax regime of 25% and Professional fees, net of tax of ₹14 Cr towards the Viatris deal
Corporate Updates
Tribute to John Shaw, Former Vice Chairman of Biocon
John Shaw, former Vice Chairman of Biocon and husband of our Chairperson, Kiran Mazumdar-Shaw, passed away on October 24, 2022, in Bengaluru. As a key member of the Board and the management team of Biocon since 1999, John Shaw has contributed majorly to the transformation of Biocon into a globally recognized, innovation-led biopharmaceutical company. In his 22 years with Biocon, he played a very important role in building the Company, ensuring the highest levels of corporate governance, as well as contributing to the financial and strategic development of the Biocon Group. He retired from the Board of Directors of Biocon on July 23, 2021, due to health reasons.
John Shaw was a man who stood tall with his values and inspired many. He was a very benevolent, erudite and a compassionate person who truly believed in philanthropy to make this world a better place. John Shaw’s vision for Biocon will continue to guide us towards our purpose of enabling equitable access to healthcare worldwide.
Board Appointment
Biocon has appointed Peter Bains as Additional Director (Category – Independent) to its Board, subject to the completion of necessary formalities under the Companies Act.
He has over three decades of experience in biopharmaceuticals, with a successful track record of building brands, businesses, teams and companies. Peter currently serves as a Non-Executive Director on the Board of Indivior PLC, a UK FTSE-listed pharmaceuticals company, as well as MiNA Therapeutics and Apterna, both privately held UK biotech companies.
Biocon Improves its 2022 ESG Score
Biocon (including Biocon Biologics) improved its ESG score to 52 from 45 in the previous year in the recently released S&P Global Corporate Sustainability Assessment report. This was accomplished on the back of key initiatives undertaken during the past year, leading to improvement in our Environmental, Social and Governance (ESG) practices, details of which were published in Biocon’s Sustainability Report 2022, which is available on www.biocon.com
ESG is at the core of our business purpose and responsibility. By serving patients, protecting the environment and promoting business integrity, we are reinforcing our commitment to building an equitable and viable future.
Business Highlights
GENERICS: APIs & Generic Formulations
- Q2FY23 revenue at Rs 623 Crore, up 18% (YoY) from Rs 530 Crore in Q2FY22.
Business Performance
During the quarter we launched two important products Sitagliptin and Vildagliptin in the EU, enabled by the brownfield capacity expansions undertaken at our Bengaluru and Visakhapatnam facilities.
The Company also received five product approvals across markets. In the EU, we obtained three approvals, for Posaconazole, our vertically integrated anti-fungal drug; Lenalidomide, an oncology product, and for Everolimus, used in the treatment of certain types of cancers and tumors. In the UK, we received an approval for Posaconazole. We also received approvals in the UAE for Mycophenolic acid delayed release tablets 360 mg, indicated for the prophylaxis of organ rejection in adult patients receiving kidney transplants.
Commissioning and Qualification of our immunosuppressants facility at Visakhapatnam and peptides facility at Bengaluru have been completed and process validation of batches will commence in the current quarter.
BIOSIMILARS: Biocon Biologics Ltd (BBL)
- Q2FY23 revenue at Rs 997 Crore, up 34% (YoY) from Rs 743 Crore in Q2FY22.
- Served ~5.3 million patients (MAT Sep 2022 basis)##.
Business Performance
Biocon Biologics’ YoY revenue growth was led by a strong performance of its biosimilars portfolio in advanced and emerging markets.
Continued progress on two of BBL’s own research assets, bDenosumab and bUstekinumab, which are undergoing global clinical trials, as well as other pipeline molecules, raised BBL’s R&D investments this quarter by 142% YoY to Rs 184 Crore, representing 18% of BBL revenue.
Core EBITDA (excluding R&D, forex, licensing income and mark-to-market loss on investments) stood at Rs 449 Crore, reflecting a growth of 48% YoY. Core EBITDA margin was at 46% for the quarter versus 42% last year. EBITDA for the quarter at Rs 214 Crore was impacted by higher R&D investments and non-cash foreign currency translational loss of Rs 35 Crore pertaining to Goldman Sachs’ OCD investment in BBL. Profit Before Tax and Exceptional Items stood at Rs 78 Crore.
Advanced Markets
In Q2FY23, the Viatris-led advanced markets business reported strong year-on-year growth on the back of improved performance by interchangeable bGlargine (Semglee), which reported an uptick in new prescription share of 14% and overall prescription share of 12% (week ended October 14, 2022).
- Increased uptake of Fulphila in the U.S. with market share surpassing 10%, despite increasing competition.
- Ogivri continues to be the leading bTrastuzumab brand in Canada and Australia with over 30% market share in both.
- The Company unlocked new opportunities with commercialization of key biosimilars, bBevacizumab and bTrastuzumab, in a few European markets.
- In Europe, bAdalimumab, reported a strong uptake in Germany and France.
- Biocon Biologics out-licensed 2 of its own pipeline assets, bUstekinumab and bDenosumab, to Yoshindo Inc. for commercialization in Japan.
Emerging Markets
During the quarter, the Biocon Biologics-led commercial business reported good performance of its insulins and bTrastuzumab in key LATAM and APAC markets.
- Our insulins continue to hold double-digit market share in several countries such as Malaysia, Mexico and Morocco.
- The LatAm business continued to show strong performance in Mexico, Argentina and Brazil.
- Recent tender wins for our biosimilar monoclonal antibodies portfolio in the AFMET region are expected to contribute revenues progressively from H2FY23.
Update on Viatris’ Biosimilars Business Acquisition and Serum Institute Vaccines Alliance
The transaction to acquire Viatris’ global biosimilars business is expected to conclude soon. On closing, Biocon Biologics will issue Compulsorily Convertible Preference Shares (CCPS) in the Company valued at USD 1 billion and make an upfront cash payment of USD 2 billion to Viatris. To fund the cash component of the deal, Biocon Biologics has secured USD 1.2 billion of debt. The balance will be funded through an equity infusion of USD 650 million by Biocon and USD 150 million by Serum. Biocon will fund USD 230 million from existing reserves and USD 420 million through mezzanine financing. Biocon is in the process of securing investments to retire the mezzanine financing, post deal closure. Biocon’s stake in Biocon Biologics will be 68% post conclusion of the Viatris and Serum transactions.
The strategic alliance with Serum Institute Life Sciences (SILS) for vaccines is on track for closure by Q3FY22.
Gearing Up for Integration & Commercial Success: Leadership Appointments
Biocon Biologics has made key leadership appointments this quarter to ensure smooth integration of the acquired business and ensure commercial success, particularly in advanced markets.
The Company appointed Stephen J. Fecho, Jr. as Global Head of Supply Chain Management. Stephen will lead the end-to-end supply chain function, including the transition and integration of front-end supply chain capabilities.
It has also appointed Stephen Manzano as General Counsel for Advanced Markets. He will lead Legal, Risk, Compliance and Audit functions in the Advanced Markets as well as Global M&A and IP.
We have also on-boarded key talent in our Advanced Markets Commercial team to build Market Access and Pricing, U.S. Policy and Advocacy capabilities.
Publications
Various research papers and articles were published during the quarter. Key ones were:
- Biocon Biologics’ role in expanding affordable access to insulins in low- and middle-income countries (LMICs) was recognized by the Access to Medicine Foundation, which featured Biocon Biologics as a leading biosimilars player in its Report on Diabetes Care titled, What are pharma companies doing to expand access to insulin and how efforts can be scaled up ? published in October 2022.
- An original research article titled ‘Immunogenicity, Efficacy, and Safety of Biosimilar Insulin Aspart (MYL-1601D) Compared with Originator Insulin Aspart (Novolog®) in Patients with Type 1 Diabetes After 24 Weeks: A Randomized Open-Label Study’ was published in the peer-reviewed pharmacology journal, BioDrugs, in September 2022.
- An article titled ‘Pharmacokinetic and pharmacodynamic equivalence of Biocon’s biosimilar Insulin 70/30 with US-licensed HUMULIN® 70/30 formulation in healthy subjects: Results from the RHINE-3 (Recombinant Human INsulin Equivalence-3) study’ was published in the peer-reviewed medical journal Diabetes, Obesity and Metabolism. An article titled ‘Biosimilars and interchangeable biosimilars: facts every prescriber, payor, and patient should know. Insulins perspective’ was published in the peer-reviewed medical journal Expert Opinion on Biological Therapy.
NOVEL BIOLOGICS
Itolizumab
Our U.S.-based partner Equillium announced encouraging interim data from the EQUALISE study, evaluating Itolizumab in patients with Lupus Nephritis. The study continues to enrol patients with topline data expected in mid-2023.
In India, the DCGI approved Equillium’s application for Phase 2 clinical trials with Itolizumab in Ulcerative Colitis in October.
Bicara Therapeutics
Our Boston-based associate Bicara Therapeutics’ lead molecule BCA101, in combination with Pembrolizumab, was evaluated in front-line systemic patients with unresectable, recurrent or metastatic head and neck squamous cell carcinoma with very encouraging response rates. During this quarter, BCA101 as a monotherapy was evaluated in patients with advanced or incurable cutaneous squamous cell carcinoma who have received previous anti-PD-1 therapy.
RESEARCH SERVICES: Syngene
- Q2FY23 revenue at Rs 768 Crore, up 26% (YoY) from Rs 610 Crore in Q2FY22.
Business Performance
Syngene’s Integrated Drug Discovery platform, SynVent, continued to gain traction with the portfolio currently standing at 18 integrated programs, with more in the pipeline.
The growth in the Development Services division was led by repeat business from existing clients and an increase in the number of collaborations with emerging biopharma companies.
After a decade of partnering with Zoetis, the Company signed a long-term biologics manufacturing agreement in July 2022 which adds to Syngene’s well-established research partnerships with BMS and Amgen. The agreement will leverage recent investments in biologics infrastructure and is likely to become a key strategic relationship for the Manufacturing Services division, in the years to come. The agreement has the potential to add ~USD 500 million business over the next 10 years.
##Moving 12-month patient population (October 2021 to September 2022)
Enclosed: Fact Sheet – with Financials as per IND-AS
About Biocon Limited:
Biocon Limited, publicly listed in 2004, (BSE code: 532523, NSE Id: BIOCON, ISIN Id: INE376G01013) is an innovation-led global biopharmaceuticals company committed to enhance affordable access to complex therapies for chronic conditions like diabetes, cancer and autoimmune. It has developed and commercialized novel biologics, biosimilars, and complex small molecule APIs in India and several key global markets as well as Generic Formulations in the US and Europe. It also has a pipeline of promising novel assets in immunotherapy under development. Website: www.biocon.com; Follow-us on Twitter: @bioconlimited for company updates.
Biocon Biologics Ltd., a subsidiary of Biocon Ltd., is a unique, fully integrated global biosimilars organization. It is leveraging cutting-edge science, innovative tech platforms and advanced research & development capabilities to lower costs of biologics therapies while improving healthcare outcomes. It has a strong research pipeline of biosimilar molecules across diabetes, oncology, immunology and other non-communicable diseases. Seven molecules from Biocon Biologics’ portfolio have been commercialized in key emerging markets and advanced markets like U.S., EU, Australia, Canada, Japan. It has many firsts to its credit including the most recent U.S. FDA approval of the world’s first interchangeable biosimilar, awarded to its Insulin Glargine, which has been commercialized in the U.S. in 2021. Biocon Biologics has entered into a strategic transaction with Viatris to acquire its global biosimilars business and has also signed a strategic alliance with Serum Institute Life Sciences for vaccines (both are subject to certain closing conditions). This will enable the Company to address the inequitable access to lifesaving vaccines and biologics globally. With a team of over 5,000 people, Biocon Biologics is committed to transforming healthcare and transforming lives by enabling affordable access to millions of patients’ worldwide. Website: www.bioconbiologics.com; Follow us on Twitter: @BioconBiologics for company updates.
Forward-Looking Statements: Biocon
This press release may include statements of future expectations and other forward-looking statements based on management’s current expectations and beliefs concerning future developments and their potential effects upon Biocon and its subsidiaries/ associates. These forward-looking statements involve known or unknown risks and uncertainties that could cause actual results, performance or events to differ materially from those expressed or implied in such statements. Important factors that could cause actual results to differ materially from our expectations include, amongst other: general economic and business conditions in India and overseas, our ability to successfully implement our strategy, our research and development efforts, our growth and expansion plans and technological changes, changes in the value of the Rupee and other currency changes, changes in the Indian and international interest rates, change in laws and regulations that apply to the Indian and global biotechnology and pharmaceuticals industries, increasing competition in and the conditions of the Indian and global biotechnology and pharmaceuticals industries, changes in political conditions in India and changes in the foreign exchange control regulations in India. Neither Biocon, nor our Directors, or any of our subsidiaries/associates assume any obligation to update any particular forward-looking statement contained in this release.

