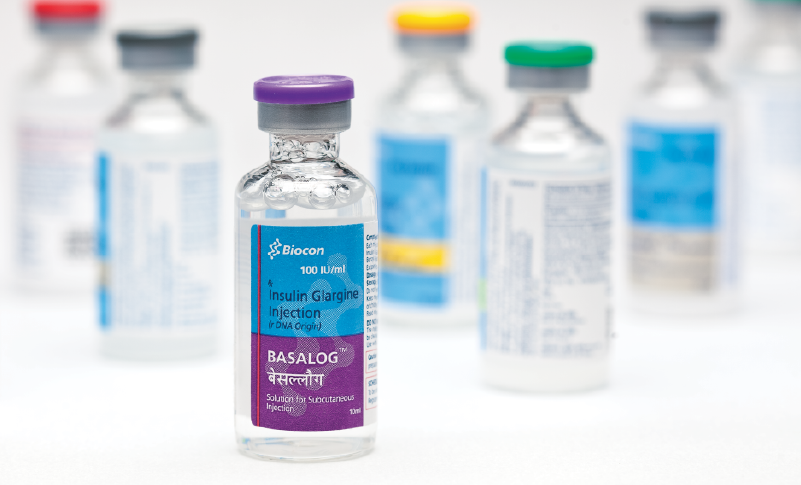
Our brand equity for over two decades, as a reliable manufacturer of complex APIs and biosimilars for global markets, has helped us build a relationship with our customer base that spans across patients, Wholesalers, Retailers, Pharmacy Benefit Managers (PBM), Health Management Organisations (HMO) and Group Purchasing Organisations (GPO).
GENERIC FORMULATIONS
Brand Equity

After launching Rosuvastatin Calcium tablets under our own label in the U.S. in 2017, we demonstrated our ability to offer reliability and affordability.
After launching Rosuvastatin Calcium tablets under our own label in the U.S. in 2017, we demonstrated our ability to offer reliability and affordability. This helped us ramp up our market share for the next two launches in the US. We have also commercialised our Rosuvastatin formulations in the UK in January 2018, through a local partner and on day one of the market formation. We are committed to cater to the requirements in both developed as well as emerging markets by leveraging our advanced R&D capabilities and cGMP compliant manufacturing facilities, including our injectable formulations and fill-finish facilities.
Cumulatively, we expect to file 15-20 applications over the next few years which include First-to-Files and Para IVs. Our investments in building commercial manufacturing scale for potent / oncology APIs and drug products will position us at the forefront of future global opportunities.

Our Generic Formulations edge
A portfolio of complex generic medicine
Ability to vertically integrate to assure continuity of supply for products with complex API needs
A strong track record on international quality compliance
Our expertise on the high potency and injectable drug product manufacturing